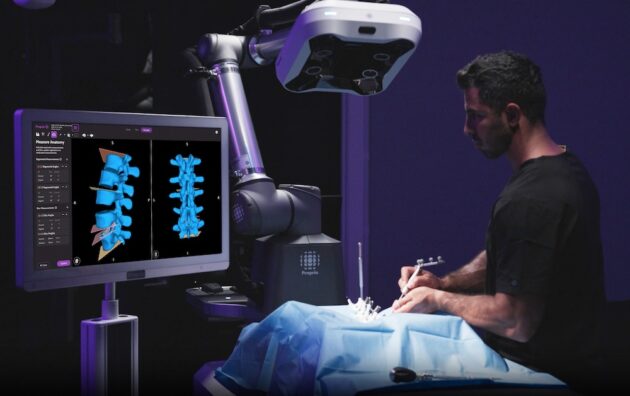
Proprio, a Seattle startup using artificial intelligence for improved surgical precision, received its second major clearance from the U.S. Food and Drug Administration to include measurements taken during operations, the company announced Tuesday.
The company’s technology platform, called Paradigm, captures high-definition images of the operating field from above and fuses them with images of pre-operative 3D scans. The system relies on advances in light field imaging, computer vision, machine learning, robotics and augmented reality.
Surgeons can assess progress against their pre-operative plans in real time during surgery, and make data-informed decisions during a procedure, reducing the need for revision surgeries, and improving patient outcomes.
“Evolving from highly educated guesswork to data-driven certainty with intraoperative measurements is game-changing,” said Gabriel Jones, CEO and co-founder of Proprio, in a statement.
The technology has been used in hundreds of surgical cases, including at leading healthcare institutions and spine centers such as UW Medicine and Duke Health.
“We’ve been using navigation in spine surgery for a while, but what this technology allows us to do is precisely find the right angle without intraoperative radiation and navigate it,” said Dr. Rick Bransford, a UW Medicine orthopedic surgeon at Harborview Medical Center. “The system can kind of tell us the trajectory and guidance to place screws with zero radiation. This is a big deal, as almost all other forms of navigation require some component of intraoperative radiation.”
Founded in 2016 and spun out of the University of Washington, Proprio employs more than 50 people and has raised $84 million to date. The company is No. 17 on the GeekWire 200 ranked index of Pacific Northwest startups.
Proprio received a previous 510(k) clearance from the FDA in 2023. The company declined to share revenue metrics.
Read the full article here